As a science enthusiast, I’ve always been fascinated by the intricate world of atoms and their variations, known as isotopes. While studying chemistry, I found myself grappling with isotope calculations, realizing that they were essential for understanding various chemical and nuclear processes. It wasn’t until I stumbled upon a practice set with an answer key that I truly grasped the concepts and gained the confidence to tackle them. This experience sparked a desire to create this resource for others, providing a step-by-step solution to a common set of practice isotope calculations, along with explanations to aid understanding.

Image: brainly.com
This comprehensive guide aims to unravel the mysteries of isotope calculations, offering a clear path toward mastery. You’ll delve into the fundamental principles governing isotopes, understand how to calculate their relative abundance, and explore their vast applications across different scientific fields. Join me on this journey as we uncover the intriguing world of isotopes and their indispensable role in scientific discovery.
Understanding Isotopes: The Building Blocks of Variation
At the heart of this exploration lie isotopes, variations of the same element that differ in their neutron count. While all atoms of a specific element boast the same number of protons (defining their atomic number), the number of neutrons can vary, resulting in isotopes. For example, consider carbon, with an atomic number of 6, indicating 6 protons in its nucleus. However, carbon exists in two common isotopes: carbon-12 (6 protons, 6 neutrons) and carbon-14 (6 protons, 8 neutrons). These variations in neutron count affect the mass of the atom, hence the distinction in their atomic mass numbers (12 and 14, respectively).
The concept of atomic mass is central to isotope calculations. While atomic mass is often represented as a weighted average, this average doesn’t truly reflect the mass of each individual isotope. Instead, it represents the average mass of all naturally occurring isotopes of that element, considering their relative abundance. Understanding these nuances is crucial for accurate calculations related to isotopic composition.
Delving Deeper: Isotope Calculations Explained
Now, let’s delve into the practical aspect of isotope calculations. Imagine you’re presented with a scenario involving isotopes: a sample containing various isotopes of an element, each with a specific percentage abundance. Your task is to calculate the average atomic mass of the element based on this isotopic composition. This scenario provides a perfect platform to illustrate the steps involved in such calculations.
The first crucial step is to gather information about the isotopes involved. Identify the element in question, note down its atomic number, and determine the mass number of each isotope. Next, you’ll need the relative abundance of each isotope, usually expressed as a percentage. With this information in hand, you’re ready to perform the calculation.
The process of calculating average atomic mass involves a simple multiplication and summation. For each isotope, multiply its mass number by its relative abundance (expressed as a decimal). Then, add up these individual products. The final sum represents the average atomic mass of the element, taking into account the contributions of all its isotopes. This calculation process serves as a fundamental tool for understanding and interpreting the isotopic composition of elements.
Applications of Isotope Calculations: A Spectrum of Uses
The applications of isotope calculations extend far beyond textbook exercises. They play a crucial role in various scientific disciplines, ranging from medicine to geology and beyond. In medicine, for instance, isotopes find their way into diagnostic imaging techniques, allowing clinicians to visualize specific organs and tissues within the body. Radioactive isotopes, like iodine-131, are used in thyroid scans to assess thyroid function. This application demonstrates the remarkable ability of isotopes to provide valuable insights into human health.
Another fascinating field where isotope calculations are indispensable is geology. Geologists use isotopes to unravel the history of rocks and fossils, determining their ages and understanding geological processes that have shaped our planet. For instance, carbon-14 dating is a well-known technique used by archaeologists to determine the age of ancient artifacts, shedding light on human history and ancient civilizations. This example showcases how isotopes serve as invaluable tools for unraveling the mysteries of our past.
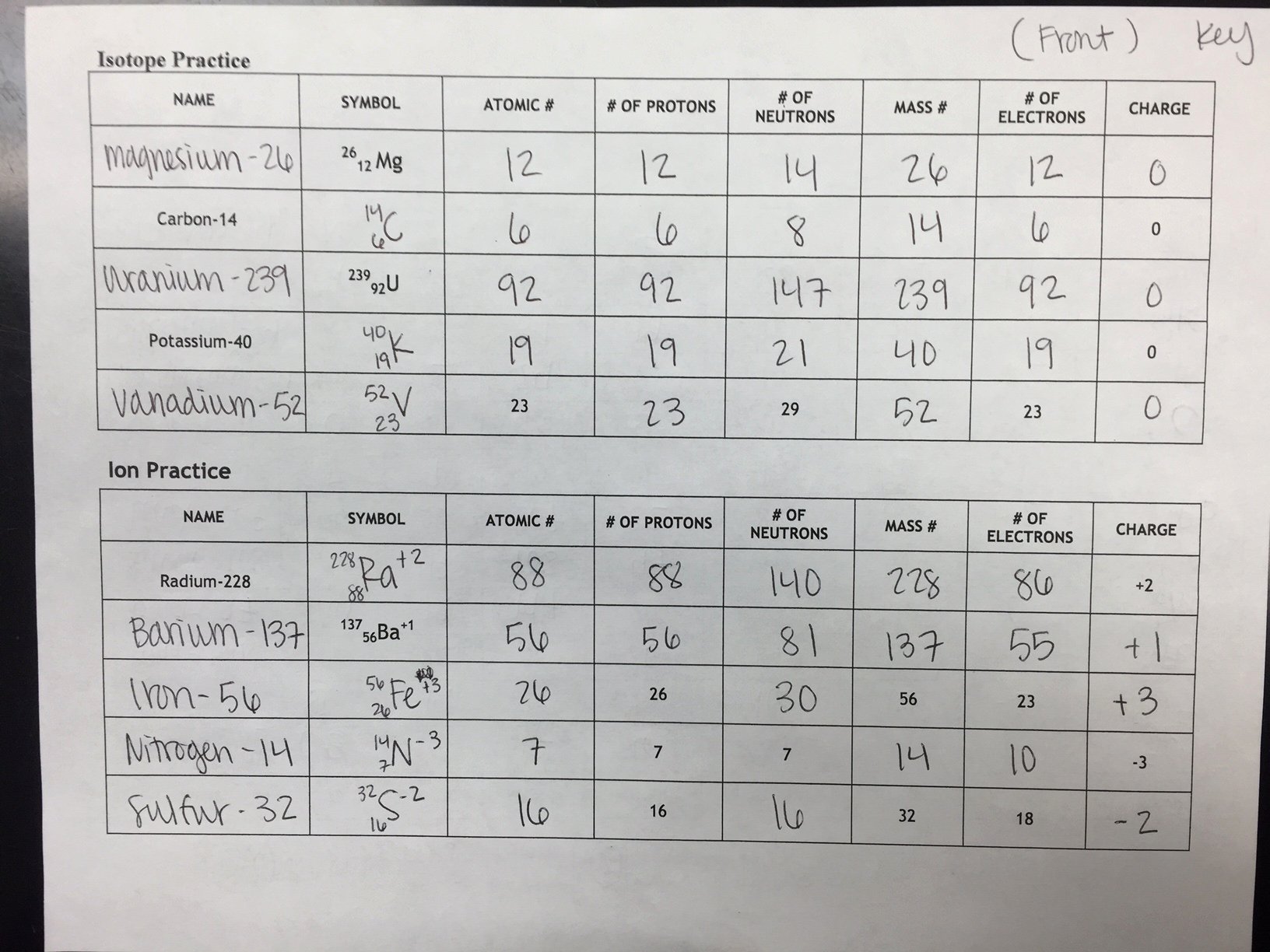
Image: lessonlibrarymoss55.z19.web.core.windows.net
Mastering Isotope Calculations: Tips and Expert Advice
Now, let’s shift our focus to practical tips and expert advice for mastering isotope calculations. The first piece of advice is to thoroughly understand the concept of isotopes themselves. Make sure you grasp the idea of variation in neutron count, how this variation affects atomic mass, and the relationship between atomic number and atomic mass.
Next, practice consistently. Approach isotope calculations like a new skill that requires constant practice to solidify your understanding and improve your speed and accuracy. Start with simple examples and work your way up to more complex ones, gradually building confidence in your abilities. Don’t hesitate to consult references, utilize online resources, and engage with peers for assistance and clarification.
FAQs: Resolving Your Isotope Calculation Queries
Q: What exactly is an isotope, and why are they important?
A: An isotope is a variation of an element with the same number of protons (atomic number) but different numbers of neutrons. They’re crucial because they have distinct mass numbers and often exhibit unique nuclear properties, leading to diverse applications in various fields, like medicine, geology, and nuclear engineering.
Q: How do I calculate the average atomic mass of an element given its isotopic composition?
A: To calculate the average atomic mass, multiply the mass number of each isotope by its relative abundance (expressed as a decimal). Then, sum up these individual products. The resulting sum represents the average atomic mass of the element.
Q: Are there any specific resources I can use to practice isotope calculations?
A: Absolutely! Numerous online resources, textbooks, and educational websites offer practice problems and answer keys for isotope calculations. Search for “isotope calculations practice problems” or “isotope calculations worksheets” to access a wealth of learning materials.
Q: Why are isotope calculations relevant to everyday life?
A: Isotope calculations are indirectly relevant to our everyday lives through various applications. Radioactive isotopes are used in medical imaging, radiation therapy, and even food preservation. Understanding isotopic composition helps in evaluating the safety of food products, tracing the origins of materials, and gaining insights into environmental processes. These applications, though seemingly unconnected to our daily routines, have a significant impact on our health, environment, and overall well-being.
Practice Isotope Calculations #1 Answer Key
Conclusion: Embark on Your Isotope Journey
In conclusion, understanding isotope calculations is a key to unlocking a world of scientific knowledge and applications. Through consistent practice, exploration of diverse applications, and a firm grasp of the fundamentals, you can navigate this intriguing realm with confidence. So, are you ready to embark on a journey of discovery and mastery in the world of isotope calculations?






