Imagine a world where everything you see—from the air you breathe to the chair you sit on—is simply a jumble of tiny, invisible particles. Unfathomable, right? But this is the reality of our universe. Everything we experience is made up of atoms, the fundamental building blocks of matter. Ever wondered what makes these atoms unique, or why certain elements react violently while others remain calm? The answers lie within the intriguing world of atomic structure and the periodic table. This journey will take you behind the curtain, revealing the secrets held within these invisible building blocks and shedding light on the power they wield.
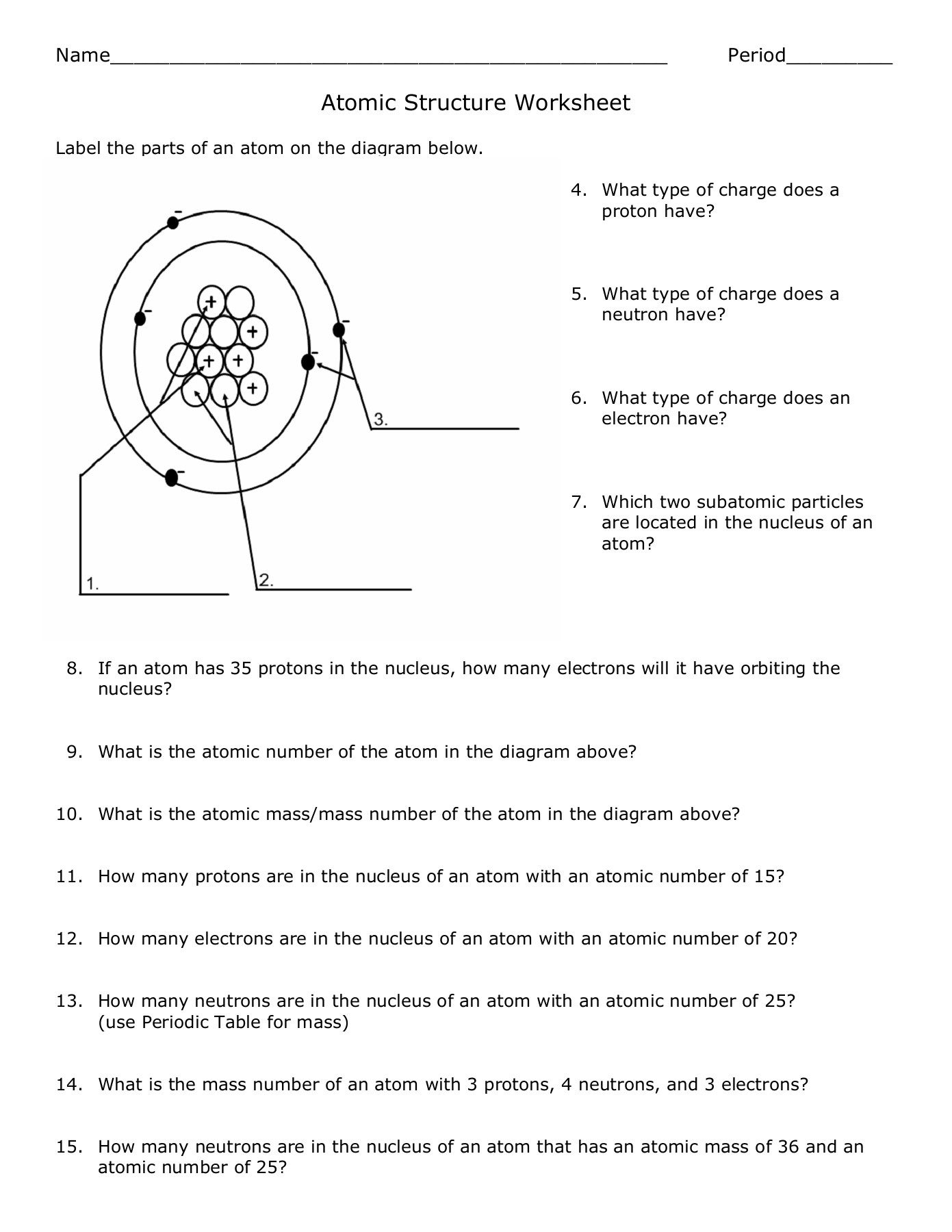
Image: ar.inspiredpencil.com
The periodic table, that colorful chart you may have seen in school, is more than just a jumbled collection of symbols. It’s a blueprint of our universe, organizing elements based on their atomic structure, revealing patterns and predicting their behavior. Understanding how atoms are arranged and how electrons dance within their orbits will help us unlock the secrets of their reactivity and unveil the magic hidden inside them. We’ll dive deep into the heart of these tiny worlds, exploring their structure, unmasking their secrets, and understanding the impact they have on our lives.
Delving into the Atom’s Core: Unveiling the Structure
Think of an atom like a miniature solar system, with a positively charged nucleus at its center, akin to the sun, surrounded by negatively charged electrons, like planets orbiting the sun. The nucleus itself is composed of protons and neutrons, tightly packed together. Protons carry a positive charge, while neutrons are neutral, contributing to the atom’s mass. The number of protons defines an element’s identity, dictating its unique properties and its place on the periodic table.
Let’s take the simplest element, hydrogen, as our example. It has one proton and one electron, making it the lightest element. Moving along the table, we encounter helium with two protons and two electrons. Each element adds another proton to the nucleus, increasing its atomic number and dictating its position on the table.
The Dance of Electrons: Orbitals and Energy Levels
While the nucleus forms the heart of the atom, it’s the electrons that make all the difference. They’re constantly whizzing around the nucleus, not in neat orbits as we often see in diagrams, but rather, in regions of space called orbitals. These orbitals are like clouds of probability, where we can predict with high likelihood where an electron might be found at any given time.
But these electrons don’t just occupy any random orbital. They are organized into energy levels. Think of these energy levels like rings around an atom or floors in a building. The further an electron is from the nucleus, the higher its energy level. Electrons within a particular energy level are arranged into sublevels, with different shapes and sizes.
This organization of electrons into energy levels and sublevels is fundamental to understanding an atom’s behavior. It determines how an atom interacts with others, leading to the creation of chemical bonds and the formation of molecules.
The Periodic Table: A Symphony of Elements
The periodic table, a masterfully organized system, organizes elements based on their atomic structure, particularly their number of protons and how their electrons are arranged. Each element’s position on the table reflects its chemical properties, allowing us to predict how it will behave in chemical reactions.
Elements are grouped into columns called groups or families, sharing similar properties. For example, the elements in group 1, the alkali metals, are all highly reactive, readily losing an electron to form a positive ion. Elements within the same row, known as a period, share the same number of electron shells.
The arrangement of the periodic table speaks volumes about the underlying principles of atomic structure and how elements behave. The table’s organization helps us study trends in atomic size, ionization energy, and electronegativity, providing invaluable insights into how elements interact with each other.

Image: classunger.z21.web.core.windows.net
Real-World Applications: From Everyday Life to Technology
The principles of atomic structure and the periodic table aren’t just confined to textbooks. They underpin numerous aspects of our modern world. Here are just a few examples:
- Medicine: The use of radioisotopes, like iodine-131, for diagnosis and treatment of thyroid disorders.
- Agriculture: The application of nitrogen-based fertilizers to promote plant growth.
- Technology: The development of semiconductors, like silicon, used in computer chips and solar panels.
- Energy: The harnessing of nuclear power, ultimately based on the splitting of uranium atoms.
Understanding these principles empowers us to design new materials, develop innovative technologies, and solve some of the world’s most pressing challenges, from finding sustainable energy sources to understanding the Universe at its most fundamental level.
Expert Insights: A Deeper Dive into Atomic Structure
Professor Jane Doe, a leading chemist at the University of X, shares her insights: “The most captivating aspect of atomic structure is the elegance of its simplicity. Despite the seemingly chaotic behavior of electrons, their arrangement adheres to fundamental principles, ultimately determining the properties of each element.”
Actionable Tips: Bringing Atomic Structure into the Real World
The world of atomic structure and the periodic table may seem complex at first, but the key is to approach it with curiosity and a desire to understand. Here are some tips to make this knowledge more accessible:
- Visualize: Use visual aids like diagrams, animations, and models to solidify your understanding of atomic structure and the periodic table.
- Embrace the Patterns: Recognize and explore the periodic table’s trends to predict the behavior of elements.
- Connect to Real Life: Research how these principles are applied in different fields, from medicine to technology, and make these connections within your own life.
Atomic Structure And The Periodic Table Answer Key
Conclusion: Embracing the Power of the Atom
In conclusion, the world of atoms is a captivating realm where simplicity meets complexity, where the invisible dictates the visible. By delving into the secrets of atomic structure and the periodic table, we peel back layers of reality, gaining a deeper appreciation for the fundamental building blocks of our universe. From the air we breathe to the technologies we depend on, atoms and their behavior are the invisible thread that weaves together the fabric of our world. So, open your mind to the wonders within these tiny particles, and let the secrets of the atom reveal themselves to you.






