Imagine a world without smartphones, computers, or even the most basic medicines. This unimaginable reality might be our fate if it weren’t for the groundbreaking work of Dmitri Mendeleev and his legendary lab in 1869. It was here, amidst bubbling beakers and swirling fumes, that the foundation of modern chemistry was laid – the Periodic Table of Elements. But how exactly did Mendeleev organize this seemingly chaotic collection of elements? What secrets did his 1869 lab hold? Let’s delve into the fascinating story of Mendeleev’s lab and the answer key that unlocked the mysteries of the universe.

Image: flyingcolorsscience.com
The year is 1869, and Dmitri Mendeleev, a Russian chemist, is facing a daunting task: organizing the known chemical elements in a meaningful and systematic way. While others had attempted this feat before, their efforts were messy and lacked a unifying principle. Mendeleev, however, approached the challenge with a unique perspective, guided by the belief that there must be an underlying order to the elements’ properties.
The Birth of the Periodic Table: A Eureka Moment in Mendeleev’s Lab
Legend has it that Mendeleev spent days and nights poring over data, scribbling down properties and atomic weights on cards, arranging and rearranging them in a desperate attempt to find a pattern. One fateful night, during a period of intense mental toil, the answer came to him in a dream. In a flash of inspiration, Mendeleev realized that the elements’ properties were not random but rather followed a recurring cycle. He immediately set to work, meticulously arranging the elements in rows and columns, placing those with similar properties together. This groundbreaking arrangement, the Periodic Table, became the fundamental tool for understanding the structure and behavior of matter, forever changing the landscape of chemistry.
Mendeleev’s 1869 lab wasn’t just a place of experimentation but also a reflection of his genius. It was filled with an array of tools and instruments that reflected the scientific methods of the era – Bunsen burners, test tubes, balances, and meticulously labeled jars of chemicals. Within this rather ordinary-looking lab, Mendeleev conducted numerous experiments, gathering data and refining his observations, all leading towards the final revelation – the Periodic Table.
The Power of Prediction: Mendeleev’s Bold Moves
The true brilliance of Mendeleev’s work lies not just in the arrangement of the known elements but also in his daring predictions. He dared to leave gaps within his table, confidently stating that these empty spaces represented yet undiscovered elements. He even predicted the properties of these missing elements based on the patterns he observed in the table. His predictions turned out to be remarkably accurate, with the discovery of gallium, scandium, and germanium later confirming his genius. This prophetic ability cemented Mendeleev’s legacy as a visionary scientist who transformed chemistry from a descriptive science to a predictive one.
While the 1869 lab of Mendeleev is long gone, its impact on scientific progress is undeniable. The Periodic Table, his most enduring legacy, became a foundational tool for scientists worldwide, providing a framework for understanding the world around us. It has enabled the development of new materials, advanced technologies, and crucial life-saving medicines. The legacy of Mendeleev’s lab extended far beyond its walls, forever shaping the future of chemistry and influencing countless scientific discoveries.
Exploring the Periodic Table: A Journey Through the Elements
The Periodic Table is a fascinating journey through the building blocks of the universe. Each element, from the familiar oxygen we breathe to the exotic plutonium used in nuclear reactors, has its own unique story. Understanding the table allows us to appreciate the interconnectedness of matter, how elements combine to form the molecules that make up our world.
The Periodic Table, in its modern form, is a testament to the collaborative efforts of scientists throughout history. It has been continuously refined and expanded with the discovery of new elements, adding further layers of complexity to our understanding of the universe. Even today, the Periodic Table continues to be a source of wonder and inspiration, inspiring scientists to unravel the mysteries of matter and the laws that govern it.
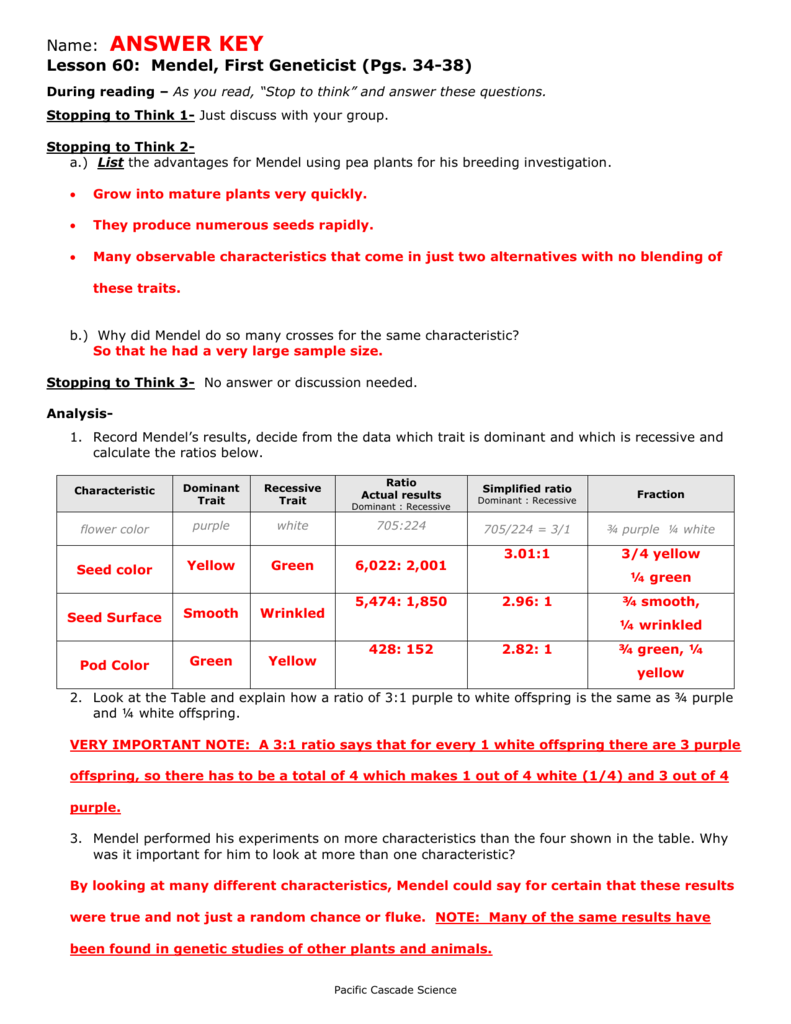
Image: wordworksheet.com
Tips for Understanding the Periodic Table
Navigating the Periodic Table can seem daunting at first, but with a few helpful tips, it can become a fascinating and rewarding adventure. Here are some key pointers for unlocking the secrets of this powerful tool:
- Start with the basics: Familiarize yourself with the fundamental concepts of atomic number, atomic mass, and chemical symbols.
- Explore the patterns: Notice the recurring trends in the table, such as the increase in atomic number as you move across and down the table. These patterns can offer clues to the elements’ properties.
- Focus on the groups: Understand the concept of groups, or vertical columns, which represent elements with similar properties. For example, the halogens (Group 17) are all highly reactive nonmetals.
- Use online resources: The internet provides a wealth of information about the Periodic Table, including interactive versions, animations, and detailed explanations of each element.
By following these tips and immersing yourself in the wonders of the Periodic Table, you can gain a deeper appreciation for the science that shapes our world. It’s a journey of discovery that can ignite a passion for science and equip you with the tools to explore the universe of matter.
FAQs about the Mendeleev Lab of 1869
Here are some frequently asked questions about Mendeleev’s lab and its influence on the Periodic Table:
Q: What were the key tools and instruments used in Mendeleev’s lab?
A: Mendeleev’s lab was equipped with common scientific instruments of the time, including Bunsen burners, test tubes, balances, and beakers. These tools allowed him to conduct experiments, analyze chemical reactions, and measure the properties of elements.
Q: How did Mendeleev’s predictions about undiscovered elements come true?
A: Mendeleev predicted the existence and properties of several undiscovered elements based on the patterns he observed in his Periodic Table. These predictions were later confirmed with the discovery of elements like gallium, scandium, and germanium.
Q: How has the Periodic Table evolved since Mendeleev’s initial work?
A: The Periodic Table has been continuously refined and expanded with the discovery of new elements, including synthetic elements created in laboratories. This evolution reflects our growing understanding of the universe and the building blocks of matter.
Mendeleev Lab Of 1869 Answer Key
Conclusion: A Legacy of Discovery and Inspiration
Mendeleev’s lab of 1869 stands as a testament to the power of scientific inquiry and the importance of pursuing knowledge. His breakthrough invention, the Periodic Table, provided humanity with an indispensable tool for understanding the world around us, paving the way for countless scientific advancements. By understanding the history and principles of the Periodic Table, we not only gain a deeper appreciation for the universe but also unlock a world of scientific possibilities.
Are you fascinated by the mysteries of the Periodic Table? Are you curious about the elements that make up our world? Share your thoughts and questions in the comments below. Let’s continue this journey of discovery together.






