Imagine a tiny, invisible world within every object you can see, a universe of swirling energy and particles so small that they defy our senses. This is the realm of the atom, the building block of all matter. For centuries, scientists have wrestled with the fundamental question: what is an atom, and how does it work? The journey to understand the atom has been a fascinating voyage through time, marked by groundbreaking discoveries, ingenious experiments, and sometimes, brilliant wrong turns.

Image: www.pinterest.com
Understanding the atomic model is crucial for comprehending the world around us. It explains the properties of materials, drives advancements in fields like medicine, technology, and energy, and allows us to unlock the secrets of the universe. Let’s embark on this incredible journey through time, tracing the evolution of the atomic model from its ancient beginnings to the mind-bending complexities of quantum mechanics.
Ancient Speculations: Seeds of the Atomic Idea
The Philosophers of Greece:
The earliest ideas about the atom can be traced back to ancient Greece, where philosophers like Democritus and Leucippus proposed that matter was composed of indivisible particles they called “atomos” (meaning “uncuttable”). While their ideas were based on philosophical reasoning rather than scientific experimentation, they laid the groundwork for future discoveries.
Aristotle’s Influence:
However, the influential philosopher Aristotle, who believed in the continuity of matter, opposed the atomic theory. His views held sway for centuries, eclipsing the early atomic ideas. It was not until the 1800s that scientific experiments began to rekindle interest in the atom.
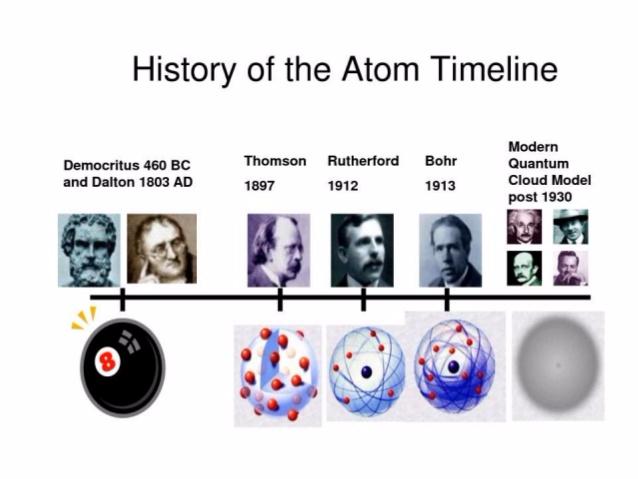
Image: quizizz.com
The Dawn of the Modern Atomic Model: The Rise of Scientific Inquiry
Dalton’s Atomic Theory (1803):
The foundation for the modern atomic theory was laid by John Dalton, an English chemist and physicist. His atomic theory, based on meticulous experimentation, revolutionized our understanding of matter. Dalton’s key postulates included:
- All matter is composed of atoms, which are indivisible and indestructible.
- Atoms of a given element are identical in mass and properties.
- Atoms of different elements vary in mass and properties.
- Atoms combine in simple whole-number ratios to form compounds.
Dalton’s atomic theory provided a framework for explaining chemical reactions and the properties of substances, effectively reviving the idea of the atom after centuries of neglect.
Thomson’s Plum Pudding Model (1897):
The discovery of the electron by J.J. Thomson in 1897 marked a turning point in our understanding of the atom’s structure. Thomson proposed the “plum pudding model,” which pictured the atom as a sphere of positively charged material with negatively charged electrons embedded throughout, as if plums were scattered within a pudding. This model was the first to suggest that the atom was not just a solid, indivisible particle but had a complex internal structure.
The Nuclear Model: Unveiling the Atom’s Internal Structure
Rutherford’s Gold Foil Experiment (1911):
Ernest Rutherford, a student of Thomson, conducted a groundbreaking experiment that shattered the plum pudding model. He fired alpha particles (positively charged particles) at a thin sheet of gold foil. While most of the particles passed straight through, a small fraction were deflected at large angles, some even bouncing back. This astonishing result could not be explained by Thomson’s model, indicating that the atom’s positive charge was concentrated in a tiny, dense nucleus at the center, with electrons orbiting around it. Rutherford’s experiment led to the “nuclear model” of the atom.
Bohr’s Model (1913):
Niels Bohr, building on Rutherford’s model, proposed that electrons orbit the nucleus in specific energy levels or shells. This model explained the emission and absorption of light by atoms (atomic spectra) and provided a more detailed picture of how electrons were distributed within the atom. However, Bohr’s model was still limited in its ability to describe the behavior of electrons.
The Quantum Mechanical Model: The Atom in a New Light
The Quantum Revolution:
The early 20th century witnessed a revolution in physics with the advent of quantum mechanics. This new understanding of physics, which described the behavior of matter at the atomic and subatomic levels, greatly impacted our understanding of the atom. In this framework, electrons are not considered as particles orbiting the nucleus but rather as probability clouds, where the probability of finding an electron at a particular point in space is represented by a wave function. This model replaced the simple, deterministic view of electrons with a more probabilistic and complex picture.
The Schrödinger Equation:
Erwin Schrödinger’s groundbreaking work, the development of the Schrödinger equation, provided a mathematical framework for describing the behavior of electrons in atoms. This equation, along with other principles of quantum mechanics, forms the basis of the modern quantum mechanical model of the atom. This model, while highly abstract and mathematically complex, has been crucial in explaining the diverse properties of elements and their interactions in chemical reactions.
The Limitations and Future of Atomic Models
Beyond the Standard Model:
Although our understanding of the atom has progressed significantly, it is not without limitations. The standard model of particle physics, which describes the fundamental particles and forces of nature, does not explain some observed phenomena, such as the existence of dark matter and dark energy. New theories are being developed to address these limitations and delve deeper into the mysteries of the universe.
The Quest for Unification:
One of the most significant challenges in modern physics is the quest for a unified theory that explains all fundamental forces of nature, including gravity, electromagnetism, and the strong and weak nuclear forces. The discovery of the Higgs boson in 2012 was a major breakthrough in this quest, but much work remains to be done. Understanding the atom, its structure, and its interactions remains a fundamental challenge in modern science, with enormous implications for technology and our understanding of the universe.
Timeline Of The Model Of The Atom
Conclusion: A Journey of Discovery
The atomic model has evolved over centuries, from ancient philosophical speculations to the complex quantum mechanical model we use today. This journey has been driven by scientific curiosity, ingenious experiments, and bold theoretical leaps. As we continue to explore the realm of the atom, we can expect new discoveries, refined models, and a deeper understanding of the universe we inhabit. The world of the atom, once a realm of speculation, has become a cornerstone of modern science, revealing the fundamental building blocks of our universe. And as we journey into the future, we can be sure that the atom will continue to surprise and enlighten us with its secrets and complexities.






