Have you ever wondered what makes up the world around us? From the air we breathe to the water we drink, everything is composed of tiny particles called atoms. These atoms are the fundamental building blocks of matter, and their arrangement and properties are beautifully summarized in a chart known as the periodic table.

Image: iperiodictable.com
Understanding the periodic table is crucial for anyone interested in science, whether you’re a student learning about chemistry or a curious individual eager to explore the wonders of the natural world. This comprehensive guide will dive into the history, structure, and applications of the periodic table, offering you a printable PDF version for convenient reference.
A Journey Through History: From Ancient Philosophers to the Modern Table
The concept of fundamental building blocks of matter dates back to ancient Greece. Philosophers like Democritus and Leucippus theorized about the existence of indivisible particles they called “atomos,” meaning “uncuttable.” However, these ideas were purely philosophical and lacked scientific proof.
It wasn’t until the 19th century that a systematic approach to classifying elements emerged. In 1869, Dmitri Mendeleev, a Russian chemist, organized the known elements by increasing atomic weight and noticed recurring patterns in their properties. He arranged them in rows and columns, leaving gaps for elements yet to be discovered. His groundbreaking table, known as the periodic table, became the foundation for our understanding of the elements.
The Structure of the Periodic Table: Deciphering the Symbols and Numbers
The modern periodic table is a visual representation of 118 known elements, arranged by increasing atomic number (the number of protons in the atom’s nucleus). Each element is represented by a unique symbol, a one- or two-letter abbreviation derived from its Latin or English name.
The table is divided into rows called periods and columns called groups. Each period indicates the energy level of the outermost electrons in the atom, while each group reflects the number of valence electrons (electrons available for bonding). Elements in the same group exhibit similar chemical properties because they have the same number of valence electrons.
Horizontal Rows: Periods
Moving across a period from left to right, you encounter elements with increasing atomic number, meaning the number of protons in their nucleus increases. This also implies an increase in the number of electrons, which are arranged in distinct energy levels or shells. The further down a period you go, the higher the energy level of the outermost electrons.
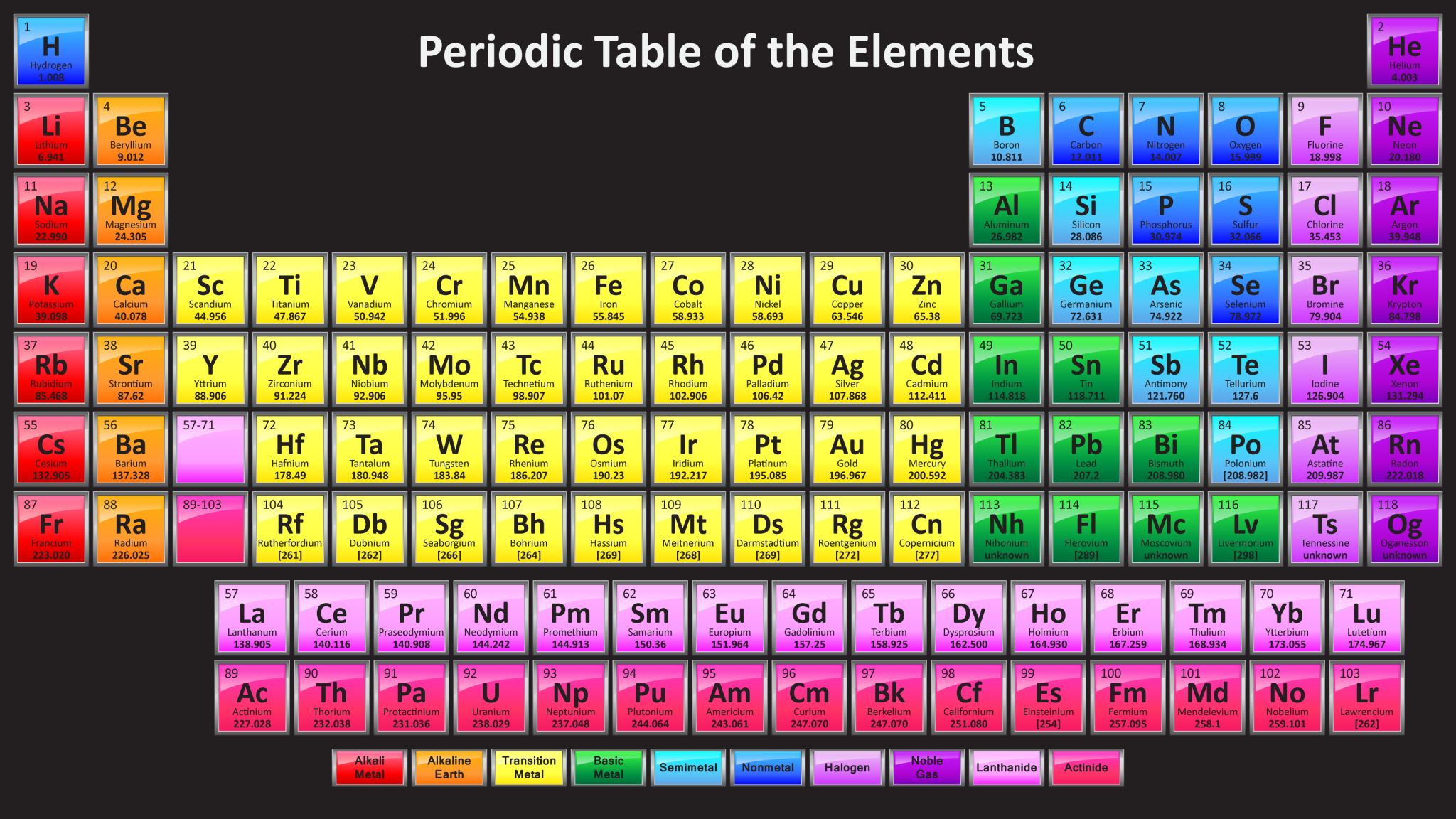
Image: eltc.gtu.ge
Vertical Columns: Groups
Each vertical column represents a group or family of elements with similar chemical properties. For example, Group 1 (alkali metals) includes lithium (Li), sodium (Na), potassium (K), and others. These elements all have one valence electron, making them highly reactive and prone to forming positive ions.
Beyond the Basics: Exploring the Periodic Table’s Secrets
The periodic table is more than just a chart of symbols and numbers; it’s a roadmap to understanding the universe. It reveals the relationships between elements, predicts their properties, and helps us understand how chemical reactions occur. It also provides vital information for fields like medicine, agriculture, and technology.
Understanding Chemical Reactivity
The periodic table helps us understand why elements react the way they do. Elements in the same group tend to react similarly because they have the same number of valence electrons. For example, halogens (Group 17) readily gain an electron to achieve a stable electronic configuration. This explains why they react violently with alkali metals (Group 1), which easily lose an electron.
Predicting Properties
The periodic table enables us to predict the properties of elements even before they are discovered. It shows trends in atomic radius, ionization energy, electronegativity, and other characteristics. For instance, elements towards the left of the table are larger in size and have lower ionization energies than those on the right.
The Periodic Table: A Printable Resource for Learning and Exploration
A printable PDF version of the periodic table is an invaluable tool for students, educators, and anyone interested in understanding the world around us. It provides a clear and concise view of the elements and their properties, allowing for quick reference and study.
When choosing a periodic table PDF, look for one that includes the following information:
- Element name and symbol
- Atomic number
- Atomic mass
- Electron configuration
- Group and period information
- Color-coding to distinguish between metals, nonmetals, and metalloids
Having a printable periodic table within reach opens up countless opportunities for learning and exploration. You can use it to delve deeper into the fascinating world of chemistry, discover the applications of different elements, or simply appreciate the beauty and complexity of the universe.
Periodic Table Of Elements With Names And Symbols Pdf
Conclusion: A Journey of Discovery Begins with the Periodic Table
The periodic table is a powerful tool that reveals the fundamental building blocks of the universe. It allows us to understand the elements, predict their properties, and explore the intricacies of chemical reactions.
Whether you’re a student seeking to excel in chemistry or a curious individual exploring the wonders of science, the periodic table provides a gateway to knowledge and discovery. With a printable PDF version at your fingertips, you can embark on your own journey to comprehend the fascinating world of atoms and elements.






