Have you ever wondered why ice floats on water? Or why a hot cup of coffee seems to “disappear” faster than a cold one? The answer lies in the fascinating concept of water density. As the temperature of water changes, its density also changes, affecting various aspects of our daily lives.
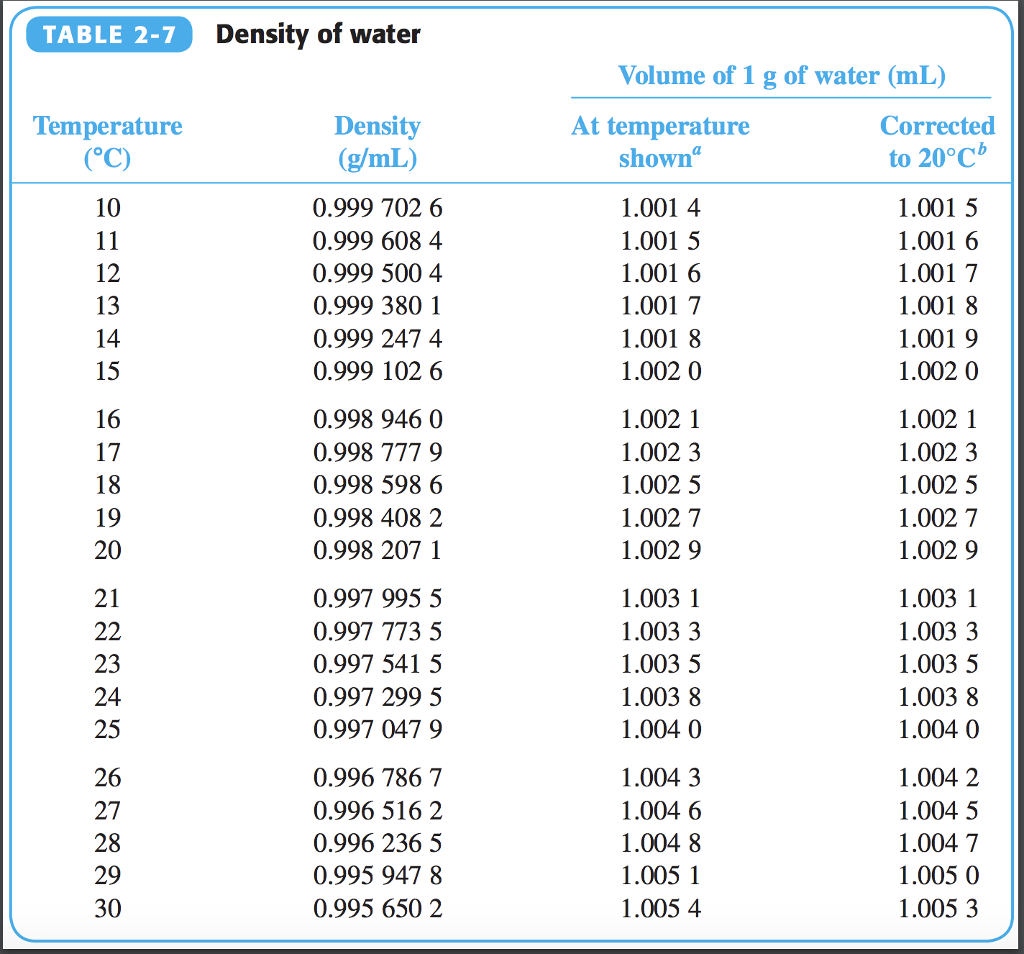
Image: quizzlibleland.z21.web.core.windows.net
Join me on a journey to understand the intricate relationship between water density and temperature. From its impact on aquatic life to its role in scientific studies, we’ll explore this intriguing phenomenon and delve into the intricacies of the water density table.
Water Density: Definition and Significance
Density is a measure of how much mass is contained within a given volume. Put simply, it describes how tightly packed the molecules of a substance are. Water, being a common and essential substance, exhibits variations in its density influenced by temperature.
The relationship between water density and temperature is crucial for understanding various natural phenomena. Here are some key aspects:
Density Changes with Temperature
As water is heated, its molecules gain kinetic energy and move more rapidly, causing them to spread out. This expansion in volume leads to a decrease in density. Conversely, as water cools, its molecules slow down, move closer together, and the density increases.
Maximum Density at 4°C
There’s a unique quirk in the behavior of water. While most substances become denser as they cool, water reaches its maximum density at 4°C (39.2°F). After this point, its density starts decreasing again as the temperature drops further. This peculiar behavior is essential for life in aquatic environments.
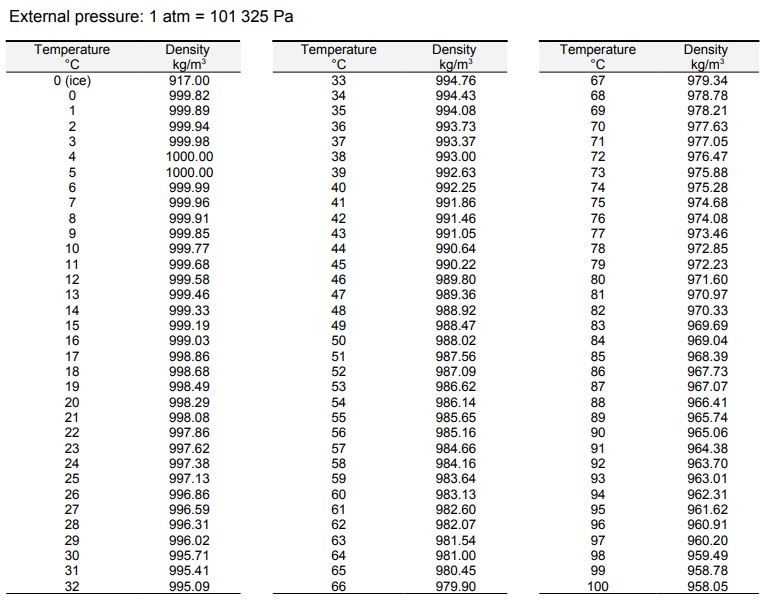
Image: materialcampusgabriele.z13.web.core.windows.net
Influence on Aquatic Life
The fact that ice floats is a direct consequence of water’s maximum density at 4°C. As lakes and oceans freeze, the coldest water at the surface forms a layer of ice, which insulates the water below from the frigid air. This allows aquatic life to survive even in harsh winter conditions.
Impact on Weather and Climate
Variations in water density play a critical role in weather patterns and climate. The density differences drive oceanic currents, which help distribute heat around the globe. These currents significantly influence regional climates, impacting rainfall, temperature, and ecological diversity.
Applications in Various Fields
Understanding water density is essential for various scientific and industrial applications. Physicists use it to study fluid dynamics, while engineers utilize it for designing cooling systems and marine structures. The food industry relies on water density principles for processing and preserving food products.
Understanding the Water Density Table
A water density table provides a numerical representation of the relationship between temperature and density. It lists the density values of water at various temperatures.
Here’s an example of a water density table:
| Temperature (°C) | Density (kg/m³) |
|---|---|
| 0 | 999.8395 |
| 4 | 1000 |
| 10 | 999.7026 |
| 20 | 998.2071 |
| 30 | 995.6503 |
| 40 | 992.2158 |
The table shows that as the temperature increases, the density of water decreases. For instance, at 0°C, water has a density of 999.8395 kg/m³, while at 40°C, the density drops to 992.2158 kg/m³.
Tips and Expert Advice for Using the Water Density Table
Here are some key tips for effectively using the water density table:
- Understand the units: The water density table usually presents data in kilograms per cubic meter (kg/m³). Ensure you understand these units and how to convert them if needed.
- Check the reference temperature: Some tables list the density at a specific reference temperature (e.g., 4°C). If your data is at a different temperature, you’ll need to adjust it based on the table.
- Consider the pressure: Density also varies with pressure. Most tables assume standard atmospheric pressure, but if you’re working under high pressure, you might need to make corrections.
- Use interpolation: If your desired temperature is not listed in the table, you can use interpolation to estimate the density based on the values available.
- Refer to reputable sources: Always use verified and reliable sources for water density data, as slight differences between tables can impact your calculations.
Understanding the intricacies of water density and effectively navigating the density table can empower you to solve complex problems across various disciplines. Whether you’re a student of physics, an engineer designing structures, or simply curious about the world around you, mastering this tool will open doors to a deeper understanding of the natural phenomena that shape our lives.
Frequently Asked Questions (FAQs)
Q: Why does water’s density change with temperature?
A: The movement of water molecules is influenced by temperature. As water gets hotter, its molecules move faster and spread out, resulting in a decrease in density. When it cools down, molecules slow down and pack closer together, making the water denser.
Q: What is the significance of water’s maximum density at 4°C?
A: It’s a unique property of water essential for aquatic life and climate systems. Because of this property, ice floats, creating a protective layer that prevents the entire body of water from freezing and allowing aquatic organisms to survive under the ice.
Q: How does the change in water density affect weather and climate?
A: Density differences drive oceanic currents. The movement of these currents helps distribute heat around the globe, influencing regional climates, rainfall patterns, and the overall global climate system.
Water Density At Different Temperatures Table
Conclusion
Water density changes significantly with temperature, a phenomenon with widespread implications in various fields. Understanding this crucial relationship is essential for accurate scientific calculations, engineering designs, and comprehending natural processes. By mastering the use of the water density table and incorporating expert advice, you can gain valuable insights into this fascinating aspect of our world.
Are you intrigued by the wonders of water density and its influence on our planet? Share your thoughts and questions below! Let’s continue exploring the mysteries of this essential substance!






