Have you ever wondered how a tiny campfire can heat a huge pot of water? Or how the sun melts ice cream on a hot summer day? These amazing phenomena are governed by the laws of heat and specific heat, concepts that play a crucial role in our understanding of the world around us. This guide will equip you with the knowledge and tools to conquer the world of heat calculations, using practical worksheets and detailed answers to solidify your understanding.
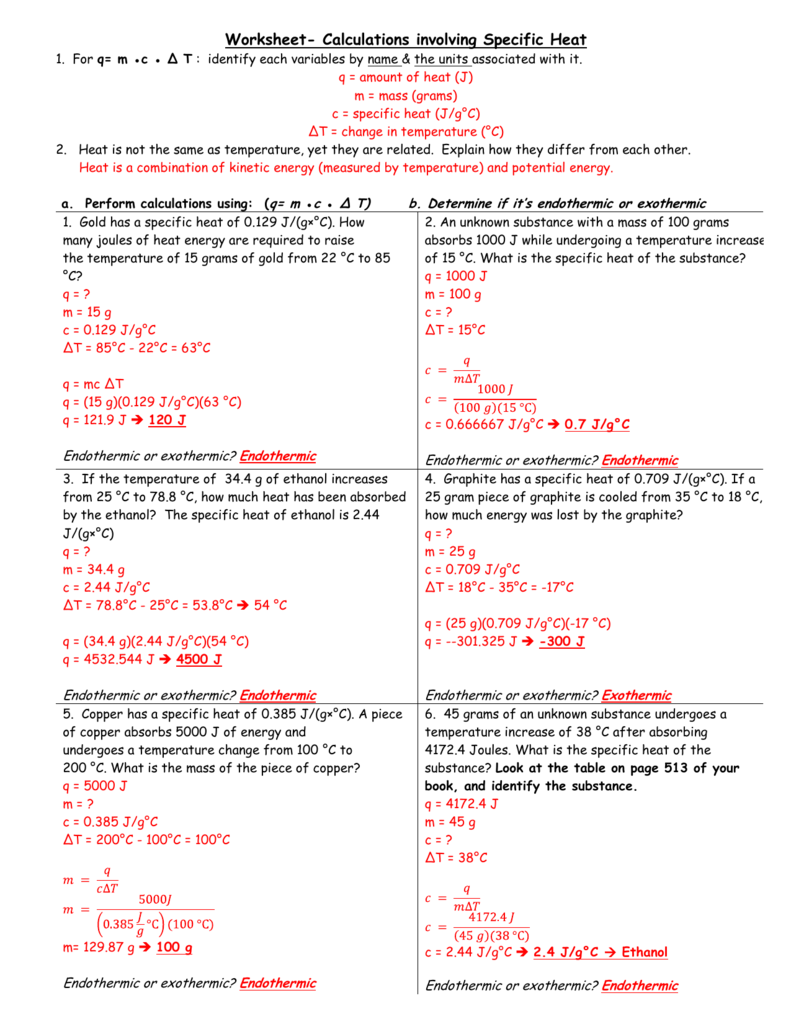
Image: lessonlibraryschulths.z21.web.core.windows.net
The journey through heat and specific heat starts with understanding the fundamental principles. Heat, in simple terms, is the transfer of thermal energy from one object or system to another. This transfer can happen through conduction, convection, or radiation. Think of it like a game of hot potato, with energy being passed from one object to another. Now, imagine a pot of water and a metal spoon inside. The spoon, being made of metal, will heat up faster than the water when you place them on the stove. This difference in heating speed is explained by specific heat, which describes a substance’s ability to absorb heat. Metals typically have low specific heat, meaning they heat up quickly, while water has a high specific heat, requiring more energy to change temperature.
Delving Deeper: Exploring the World of Heat and Specific Heat
Our exploration of heat and specific heat often involves calculations. But fear not! We’ll break down the most common equations and provide you with practice worksheets to build confidence in your understanding.
Let’s start with the most fundamental equation:
Q = mcΔT
- Q represents the amount of heat energy transferred. Think of it as the “hotness” being exchanged.
- m is the mass of the substance. The more material, the more energy it needs to change temperature.
- c is the specific heat capacity of the substance. Remember, water has a high specific heat, meaning it takes more energy to raise its temperature compared to a metal like copper.
- ΔT is the change in temperature. This is the difference between the final and initial temperatures of the substance.
Imagine a scenario where you’re heating a cup of water. You want to know how much heat energy is needed to raise its temperature from 20°C to 80°C. You have a cup of water, which weighs 250g. The specific heat capacity of water is 4.184 J/g°C. Using the formula above, you can calculate the heat needed:
Q = (250 g) (4.184 J/g°C) (80°C – 20°C) = 62,760 J
So, you would need 62,760 Joules of heat energy to raise the temperature of your cup of water from 20°C to 80°C.
Unlocking the Power of Worksheets: A Hands-on Approach to Mastery
These formulas may seem daunting at first, but practice makes perfect! Numerous worksheets are specifically designed to help you master these concepts. These worksheets offer a variety of problems, ranging from simple calculations to more complex scenarios that might involve phase changes or mixtures. Working through them step-by-step will help you build your confidence and deepen your understanding. For added convenience, you can find worksheets with answers readily available online and in textbooks.
Navigating Real-World Applications: Where Heat and Specific Heat Shine
Beyond the equations and worksheets, it’s important to recognize how these concepts apply to our everyday lives.
Think about cooking: You use the high specific heat of water to gently heat it up for soups and stews, while the low specific heat of your cooking pan allows it to heat up quickly, ensuring your food cooks faster. We use the principles of heat transfer in heating and cooling systems, from your home’s air conditioner to keeping food cold in a refrigerator.
Weather forecasting relies heavily on these concepts. Understanding how heat energy is transferred through the atmosphere allows meteorologists to predict patterns and potential weather events, including storms and extreme temperatures.

Image: zipworksheet.com
Expert Insights: Tips and Tricks for Mastering the Heat Equation
Mastering the calculation of heat and specific heat requires a systematic approach. Here are some tips from experienced educators:
- Start with the basics: Understand the definition of each term in the equation and what it represents.
- Organize your information: Write down all the values you have before attempting the calculation. This will prevent confusion and errors.
- Double-check your units: Ensure that all units are consistent before plugging them into the formula. For example, if your mass is in grams, your specific heat capacity should also be in grams.
- Practice, practice, practice!: Regularly working through worksheets and example problems will solidify your understanding and build your confidence.
Calculating Heat And Specific Heat Worksheet With Answers Pdf
Conquering the World of Heat: A Call to Action
This journey into the world of heat and specific heat is a fascinating adventure, revealing the hidden world of energy transfer and its impact on our lives. By understanding these concepts, you can become a more discerning consumer of energy and a more informed participant in discussions regarding climate change and sustainable practices.
So, embark on your journey of discovery! Explore those worksheets, delve deeper into the rich world of physics and chemistry, and embrace the power of knowledge to make a difference in the world around you.






