Ever wondered about the tiny building blocks that make up everything around us? From the air we breathe to the water we drink, everything is composed of atoms, the fundamental units of matter. But did you know that atoms can also transform into charged particles called ions? This fascinating transformation is at the heart of chemistry, and understanding it can unlock a whole new world of scientific knowledge.
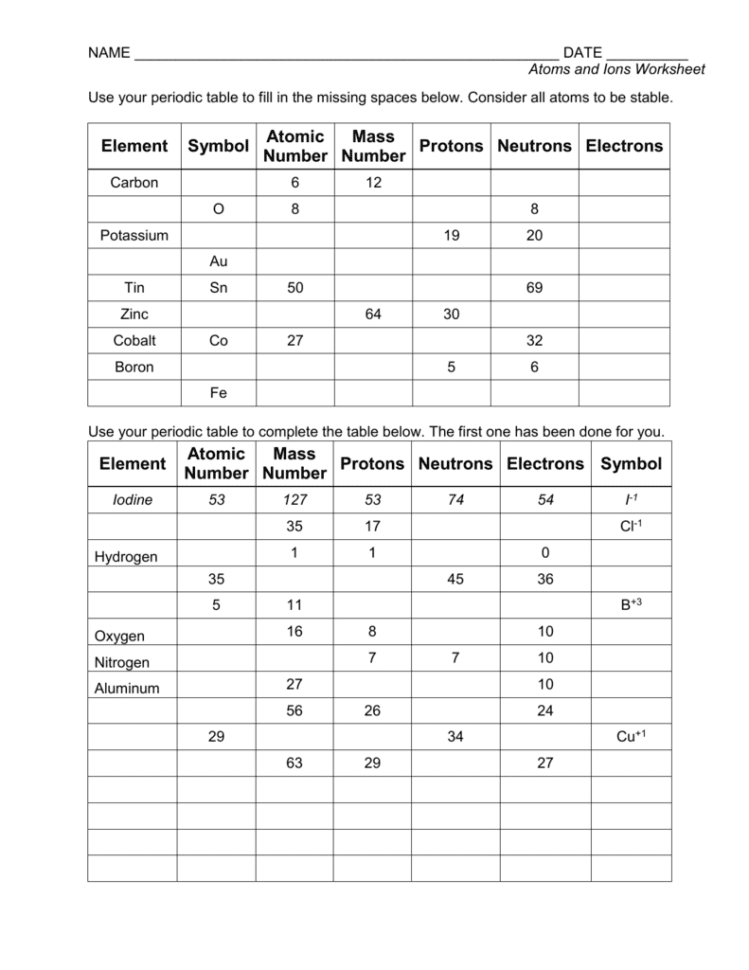
Image: db-excel.com
This article will delve into the intriguing world of atoms and ions, exploring the exciting journey of their transformation and how this knowledge impacts our understanding of the universe. We will explore the vital role of atoms and ions in our daily lives, from the electricity that powers our homes to the chemical reactions that keep us alive. So, fasten your seatbelts and get ready to embark on an exciting adventure into the microscopic world of matter.
Unveiling the Atom: The Foundation of Matter
Imagine a tiny, bustling city filled with tiny structures, constantly moving and interacting. This city, unbelievably small, represents a single atom, the fundamental unit of an element. Each atom has a central core called the nucleus, consisting of positively charged protons and neutral neutrons. Surrounding this nucleus are negatively charged electrons that orbit around the nucleus in specific energy levels.
Atoms are incredibly tiny, with diameters measured in nanometers (billionths of a meter). Despite their diminutive size, they are responsible for the vast diversity of substances in our universe. Each element, from the hydrogen in water to the iron in our blood, is defined by the number of protons in its atoms.
Atoms in Transformation: The Birth of Ions
Imagine an atom, serene in its existence, suddenly gaining or losing an electron. This seemingly minor change creates a dramatic transformation, giving birth to an ion. An ion is simply an atom that has gained or lost electrons, resulting in a net electrical charge.
When an atom gains an electron, it becomes negatively charged and is called an anion. On the other hand, when an atom loses an electron, it becomes positively charged and is called a cation. This change in charge dramatically alters the chemical properties of the atom, leading to fascinating interactions that drive various chemical processes.
The Power of Ions: Shaping Our World
Ions are the stars of many chemical reactions, playing pivotal roles in various processes that are fundamental to life and the universe. Their charged nature enables them to interact with other ions, atoms, and molecules, creating a symphony of chemical reactions that drive life’s processes and shape our world.
Here are some fascinating examples:
- Ionic Bonding: Opposite charges attract, and this principle governs the formation of ionic compounds. Cations and anions, driven by electrostatic forces, bond together to form stable compounds like table salt (NaCl), essential for human life.
- Electrical Conductivity: Ions are the key players in electrical conductivity. In solution or molten state, ions can move freely, carrying electrical charges and enabling the flow of electricity. This property is vital for various applications, from batteries to electrical circuits.
- Biological Processes: Ions play crucial roles in biological processes. Sodium (Na+) and potassium (K+) ions regulate nerve impulses, while calcium (Ca2+) ions are essential for muscle contraction and bone formation.
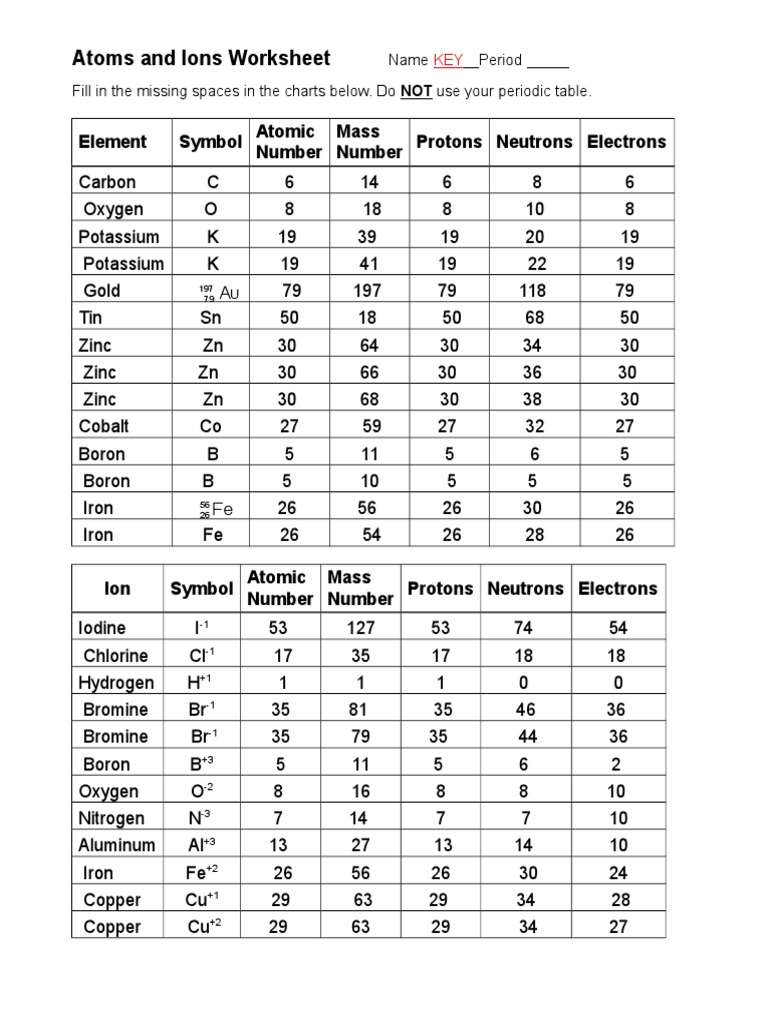
Image: www.scribd.com
Decoding the Atom vs. Ions Worksheet Answers PDF
The “Atoms vs. Ions” worksheet is a valuable tool for understanding this crucial transformation. It typically presents various scenarios, including the formation of ions from atoms, the naming of ions using their charges, and interpreting the structural differences between atoms and ions.
Completing the worksheet requires a solid grasp of atomic structures, the concept of electrical charges, and the rules for forming ions. Each question provides an opportunity for students to test their knowledge and solidify their understanding of this essential topic.
Empowering Learners with Practical Tips
Here are some tips for mastering this crucial concept and acing your “Atoms vs. Ions” worksheet:
- Visualize the Structure: Imagine a simple atom, with protons and neutrons in the nucleus and electrons orbiting around it. Visualizing the arrangement helps understand how the loss or gain of electrons leads to ion formation.
- Pay Attention to Charges: Always remember that cations are positively charged due to the loss of electrons, while anions are negatively charged due to the gain of electrons.
- Practice Naming Ions: Familiarize yourself with the rules for naming ions, including the use of Roman numerals for transition metals.
- Use the Periodic Table: The periodic table is your best friend! It provides a wealth of information about elements, including their number of protons, electrons, and their tendency to form ions.
Atoms Vs Ions Worksheet Answers Pdf
Wrapping Up: A Journey into the Microscopic World
Understanding atoms vs. ions is a journey into the microscopic world, unlocking the secrets of matter and how it shapes our universe. By comprehending the transformation of atoms into ions, we unlock the key to understanding chemical reactions, biological processes, and the intricate workings of the universe.
This article has been your guide, empowering you with knowledge and practical tips to conquer the “Atoms vs. Ions” worksheet and master this fascinating aspect of chemistry. So, continue exploring the wonders of the microscopic world, and remember, the journey of learning never ends!






